New paper on organic functionalized nanoparticles in nematic LCs
(click the above image to access the article online)
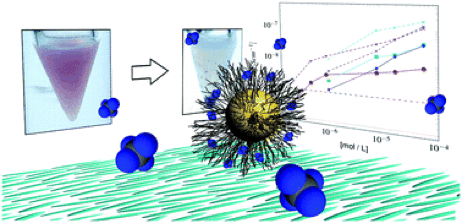
Congratulations to Martin on the publication in J. Mater. Chem. C of his dielectric spectroscopy study “Why organically functionalized nanoparticles increase the electrical conductivity of nematic liquid crystal dispersions”!
This paper gives a first systematic study of how and why nanoparticle doping raises the electrical conductivity of thermotropic liquid crystals like the commonly studied 5CB. By a careful analysis of the dielectric spectra, he shows that the hydrodynamic radius of the ionic charge carrier is much smaller than the nanoparticles, ruling out the particles themselves as the source of conductivity. The ligand molecules are also not the reason, as is demonstrated by strong sonication of the dispersions, such that the ligands detached from the nanoparticles. While this causes nanoparticle aggregation and the loss of suspension stability, the effect on conductivity is negligible. The ligand shell is, however, partially responsible, because the ions giving rise to the conductivity increase are most likely remnants from the ligand-functionalized nanoparticle synthesis process. We propose that these ions are brought in with the ligand shell when the particles are dispersed in the 5CB. Interestingly, the ions appear not to be released in an isotropic and aromatic solvent such as toluene, which is often the host for commercial gold nanoparticle suspensions, but 5CB is an ideal host for their dissolution. The aliphatic ligand shell has a higher compatibility with 5CB than with toluene, thanks to the alkyl tail of 5CB, and at the same time the high polarity of the 5CB (due to the cyano group) allows better ion dissolution than in regular hexane. Finally, the nematic order of the 5CB solvent provides an anisotropic environment in which the ligands are stretched out preferentially along the director, making release of ligand-bound ions to the solvent more likely.
/JL/






